Reprinted from "Gasohol: A Seminar. The Technical Aspects of Fuel Alcohol Production" by Stanley Parker, Biocon (U.S.) Incorporated, Lexington, Kentucky (1980).
Since the primary purpose of mashing, fermentation, and distilling is to produce the maximum amount of ethanol from a given mass of starch, whether from corn or other cereal grain, potatoes, sugar, molasses, whey, or cellulose products, it follows that growth of organisms other than distiller's yeast must be controlled. Otherwise, sugar and alcohol are lost to bacteria, which do not produce alcohol, and wild yeast which produces it less efficiently or produces acids.
TYPES OF INFECTION
Unwanted organisms such as the Acetobacter, which produces vinegar, and the Lactobacillus family, which produces lactic acid, are in the air and in the materials and equipment used to produce ethanol. They will always be with us and must be controlled in order to get maximum alcohol yields from our equipment and materials.
Infinitely small quantities of these organisms -- if present -- can reduce yield in the following ways:
[1] Acetic acid bacteria convert alcohol to acetic acid, thus reducing the alcohol yield. Exclusion of air from the fermenting or fermented mash reduces this hazard.
[2] Lactic acid bacteria take away the sugar from the yeast before and during fermentation.
Effect of infection on yield:
Infection Level (Bacteria per ml.) -- Loss in Yield (Percentage)
0-1 million -- up to 1%
I- 10 million -- 1-3%
10-100 million -- 3-5%
over 100 million -- over 5%
LOSS IN YIELD
A loss of 5%, which is quite probable under conditions favorable to bacteria growth, of course means a loss of alcohol of 5 gallons/day on a 100 gallon/day operation and 50 gallons/day on a 1,000 gallon/day operation. At a cost of $1.50/gallon in a 300 day/year operation, this amounts to around $22,000/year for the larger operation or about $2,200/year for the smaller operation.
WHERE INFECTION OCCURS
It is very difficult for infectious organisms to gain a foothold and multiply on smooth, clean surfaces. They are harbored on surfaces covered to any extent with deposits of starchy, sugary, protein-rich, or mineral-rich materials. They occur in places that are hard to clean, such as porous surfaces, cracks, sharp angles, corners, gaskets, valves, pressure gauges, in-place thermometers, and pump packing. Deposits in these areas protect the organisms in them from heat and sterilizing solutions.
Prior to boiling mash, assuming the time from commencement of mashing to boiling is not long, these bacteria are not dangerous. Storing the mash for any length of time without heating to temperatures over 180 deg F, will allow the infectious organisms to take over and use up some of the potential yield-producing materials. Distressed, damp, long standing raw materials are probably loaded with bacteria (just as water can be if it is not fresh or is from an impure or untreated source). Backset, the hot liquid separated from the spent mash at the end of distillation, which can be reused as a hot mashing-in liquid, can also harbor organisms if the temperature before use has been allowed to drop. It is necessary, therefore, to heat to boiling as soon after mashing-in as possible.
The greatest infection danger occurs at temperatures below 180 deg F before the addition of yeast at commencement of fermentation.
Therefore, the faster the cooling down is completed, the less danger of infection there is. Cooling down by standing overnight can be dangerously infectious. You will get alcohol, but the yield will almost certainly be adversely affected.
CARE AND CONTROL 0F YEAST
Once you have reached the correct temperature for fermentation to begin, normally between 85-90 deg F, the yeast should immediately be put in, or "pitched", as it is referred to in the trade, before any deleterious microorganisms can take over. Yeast should be slurried beforehand in clean, tepid water (100-110 deg F), not hot, to give it a start. If a good vigorous uncontaminated yeast is used, it will commence to "bud" or grow after a period of several hours and will greatly reduce or eliminate any danger from infectious organisms by "smothering" or outgrowing them. In this respect, it is sometimes of benefit to increase the pitching rate in order to increase the "smothering" effect. The number of yeast cells used for pitching should be about 5-10 million cells per ml. (20 drops) or 2-4 lbs. dried active distiller's yeast per 1,000 gallons of mash. The use of yeast food in a bubbling system will also help.
Depending on the starting temperature, there will be a 6-12 hour lag, after which the fermentation will start in earnest. Depending on the original percent of solids in the brew, the yeast will multiply from 5 to 20 times before the end of fermentation.
In the yeast family, Saccharomyces cerevisiae, there are many variants. For example, members of the same family, baker's and brewer's yeast, do not produce alcohol as efficiently as distiller's yeast, which produces the most alcohol in the shortest time and therefore should be used in the production of ethanol.
With good microbiological control, it may be economically worthwhile to maintain an expanded yeast culture supply in a large operation. Expanded means grown from one or several cultures of yeast cells to a sufficient amount to start a batch. In the event of contamination (defined as more than 1,000 to 2,000 bacteria per 1,000,000 yeast cells) a yeast crop can be cleansed by two methods:
[1] Acid Wash -- Recommended for slightly infected yeast, or routine use. To a slurry (0. 2% dry yeast basis) of the yeast, add enough tartaric, phosphoric, or sulfuric acid to bring the pH down to 2.2 and let stand for at least 2 hours.
[2] Acid-Oxidizing Wash -- Recommended for highly contaminated yeast, where discarding is not practical. To the yeast slurry add 0.75% by weight of ammonium or sodium persulfate. Acidify to pH 2.2 and hold for 2 hours, no more, as a longer time may weaken or deactivate the yeast ... or acidify to pH2.8 and hold for 12-24 hours.
YEAST WASHING
Acid-oxidizing wash (for infected yeast)
[1] To a slurry of the yeast solution add 0.75% by weight of ammonium or sodium persulfate.
[2] Add sufficient tartaric, phosphoric, or sulfuric acid to bring the pH down to 2.2 and let stand for 2 hours, no more, then pitch.
or [3] After adding the ammonium or sodium persulfate, add sufficient of any of the above acids to bring the pH down to 2.8 and let stand for 12-24 hours before pitching.
The effect is to disable the bacteria without affecting the yeast so that, at the start of fermentation, the yeast can take over before the bacteria have a chance to multiply. If, despite washing, attenuation (and so the efficiency of conversion of sugar to alcohol) is not satisfactory, the original yeast characteristics may have been affected ... and therefore, a fresh supply of yeast is recommended.
For the smaller distiller, a supply of fresh viable dried active distiller's yeast is mandatory. Dried active distiller's yeast will maintain 90-95 % of its activity for up to 6 months if held at 35-55 deg F but can lose 30% of its activity if held at 70 deg F for 4 months, which points out the need for holding at low temperatures and not holding yeast for more than a few months before use.
Pressed yeast, even if refrigerated, should be used in a matter of days as the presence of water causes yeast to lose its activity.
To keep the yeast operating at full potential, the following conditions should be observed:
[1] Keep the fermenting mash below 100 deg F as temperatures over this may weaken the yeast.
[2] Avoid concentrations of iron, copper, and other heavy metals. Iron should not be in too high a concentration in either the water or the raw materials, so iron and steel surfaces should be coated. A small amount of copper is good for the yeast but in large amounts it has a poisonous effect. Aluminum is without effect ... however, effective cleaning solutions may seriously erode this metal.
[3] Normally, alcohol percentages over 11-12% by volume have an adverse effect on yeast growth but by use of variants of the present strains, now being developed, it should be possible to ferment at higher alcohol levels.
[4] Normally, there is enough protein (amino acids) for yeast growth ... but sluggish, poor yielding fermentations will sometimes require additions of inorganic or organic nitrogen, such as ammonium salts, urea, or amino acids.
[5] Too low pH's from acids and other end products of bacterial growth may not only use up some of the starches and sugars, but may adversely affect the yeast's attenuative powers.
[6] It should be kept in mind that distiller's yeast ferments best at pH's between 4. 0 and 5.0, so the faster the start, the sooner the pH becomes optimum. Also, with lowering of pH, growth of bacteria is discouraged.
CONTROL OF MICROORGANISMS
These are best controlled by ensuring that all surfaces, especially those directly in the preparation and fermentation areas, are kept clean and maintained in a sanitary condition. This is made easier by proper design of equipment.
All surfaces should be smooth. This is possible with polished stainless steel, glass-lined or plastic-coated iron, and copper, with the tanks preferably in one piece. Copper must be kept shiny with use of acids (not caustic solutions) to minimize copper pickup. Wood fermenters have been used but are subject to leaking between staves and must be coated with pitch, or bacteria will nest on and below the surface. Use of pitch does not allow use of hot sanitizing solutions.
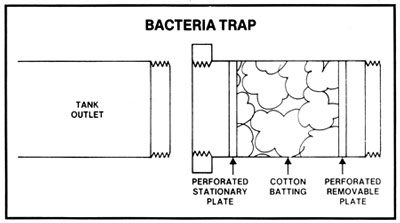
During active fermentation there is always a slight pressure on a closed fermenting vessel which does not allow microorganisms in the air to enter, but before and after active fermentation there may be air pressure fluctuations in and out of a closed tank. It may then be preferable to trap these organisms in an easily sterilized and connected trap. This bacteria trap contains cotton batting which is easily replaceable and can be sterilized before each use by placing in an oven for 1/2 hour at 250 deg F. In some geographical locations, contaminants in the air may be massive. In any case, open fermenters could be covered by sterile sheets to prevent microorganisms from falling into the fermenting mash.
As little equipment as possible should be inside all vessels, especially in cooling down and/or in the fermentation area. Here jacketed cooling is preferred but where this is not economically feasible, as few cooling coils as possible should be installed, preferably single large coils which lend themselves to easier cleaning than many small coils. Coils should also be spaced to facilitate cleaning.
CLEANING
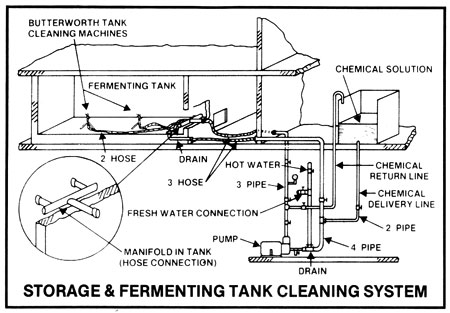
In all operations, any solids adhering to the inner surfaces of all vessels must be removed between batches or at routine intervals in continuous operations, not only to facilitate heat transfer, but to remove potential pockets of infection. In small operations, the cookers and fermenters may be pressure hosed down after use, then filled with hot (over 180deg F) water or preferably hot 2-3% caustic soda solution, left draining overnight, and again pressure rinsed down before use. The caustic soda solution may be pumped out to a holding vessel, made up to strength, and heated and re-used several times before discarding ... that is when it contains a discernibly large amount of solids. This simple system may be used for open cookers and fermenters. Hot caustic soda solutions should contain surfactants (wetting agents) to improve penetration and should be circulated for 1/2 to 1 hour.
The above system may be used for small and large operations but by far the most efficient method to maintain effective sanitation is use of cleaning-in-place or C.I.P. systems used with closed equipment.
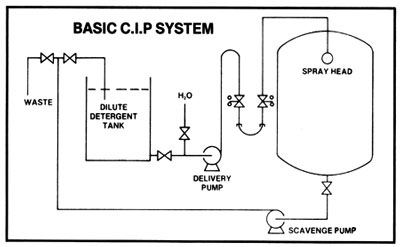
A simple C.I.P. system consists of a caustic solution tank and connections to and from the tank. Pre-rinsing and flushing out after cleaning can be done from a hose connection to the water supply. Sanitation of equipment between batches with chlorine or iodine solutions may be done from a smaller tank. Connections to and from tanks are made with a flexible hose. With the tank outlet higher than the solution tank, only one pump is needed: the pump used for transferring the mash. It must always be kept in mind that when a heated tank is cooled down, a more than sufficient supply of displacement air must be available so that collapsing of a tank, because of the vacuum formed, cannot occur.
A more sophisticated C.I.P. system is composed of a cleaning water tank, a caustic solution tank with heating device, a sanitizing solution tank, and permanent connections. Two pumps are used, one to supply the cleaning jets and the other for return from the cooker or fermenter. Automated cleaning controls may also be incorporated.
Many such rotary cleaning jets are available on the market. One such cleaning jet is fed with pressures from 50 to 160 lbs. per square inch from the cleaning system tanks, and it very effectively and quickly removes accumulated material from all surfaces by its pattern of impingement on the sides of vessels and on the internal equipment in the fermenter or vessel. Such cleaning jets are normally permanently attached to the ceiling of a tank.
A single portable cleaning-in-place device, incorporating a jet which is introduced through a bottom or top manhole with a plastic dummy door, may also be used to clean one or more tanks in succession. When pumping hot caustic solutions, care must be taken to avoid splashing and all conveying lines and equipment must be maintained in leakproof condition. It is recommended that operators wear face shields and plastic or rubber gloves.
FOAMING
When excessive foaming occurs in fermenters, this foam may enter the blowoff or C02 collection lines. In the course of time, the liquid in the lines will become infected and drip back into the fermenting mash, resulting in a partly or greatly infected mash and lowered yield. This can be eliminated by adding an antifoam solution to the mash at the start of fermentation. Addition of an antifoam results in:
[1] Reduced foam head and clean C02 blowoff lines.
[2] A smaller yeast ring above the mash at the end of fermentation brought about by the reduced foam head and, therefore, easier cleaning.
[3] The possibility of increasing the fermenting mash volume and still containing it in the fermenter.
The recommended rate of use of an antifoam is 25 parts per million or 1/4 lb. per 1,000 gallons of mash.
START OF DISTILLATION
When the reading on your saccharometer has not changed for 6-12 hours, distillation should commence. Otherwise acetic acid may start to convert the alcohol to vinegar. If a layer of carbon dioxide is maintained above the surface of the fermented mash, however, acetic acid bacterial action will not occur or will be minimal.
SANITIZERS
The following chart indicates the rates of use, etc., and the advantages and disadvantages of the various sanitizers normally used:
Characteristics of Sanitizers
Active Chlorine
Recommended Applications and Suggested Concentrations
Rate: 200 ppm*
1. C.I.P. cleaning
2. Porous surface
3. Stainless steel equipment
4. Water treatment (20 ppm*)
Advantages
1. Good germicidal efficiency
2. Nontoxic in recommended dilution
3. Fast
4. Non-film-forming
5. Ease of measurement
6. Ease of use
7. Low cost
Disadvantages
1. Toxic at shelf strength
2. Low shelf life
3. Stability in use varies with temperature
4. Poor penetration
5. Highly affected by organic matter
6. Affected by low pH
Iodophor
Recommended Applications and Suggested Concentrations
Rate: 25 ppm*
1. Aluminum equipment
2. C.I.P. cleaning
3. Hand dip, production
4. Hard water
5. High-iron water
6. Stainless steel equipment
Advantages
1. Fast
2. Good penetration
3. No or slight formation of film
4. Ease of measurement
5. Ease of use
6. No effect to skin
7. Noncorrosive to stainless steel
8. Moderate cost
Disadvantages
1. Germicidal efficiency with vegetative cells only
2. Toxicity in recommended dilution depends on wetting agent
3. Toxic at shelf strength
4. Shelf life varies with temperature
5. Moderately affected by organic matter
6. Affected by high pH
Quat
Recommended Applications and Suggested Concentrations
Rate 200 ppm*
1. Bacteriostatic film
2. Presence of organic matter
3. Porous surface
Advantages
1. Excellent shelf life
2. Excellent stability in use
3. Fast
4. Excellent penetration
5. Low effect by organic matter
6. No corrosiveness
7. Moderate cost
Disadvantages
1. Selective germicidal efficiency
2. Moderately toxic in recommended dilution ... depends on wetting agent
3. Toxic at shelf strength
4. Forms film
5. Affected by other water constituents
6. Produces high foam
Acid Sanitizer (Anionic)
Recommended Applications and Suggested Concentrations
Rate: 130 ppm*
1. C.I.P. cleaning
2. Prevention of film formation
3. Hard water
4. Stainless steel equipment
Advantages
1. Good germicidal efficiency
2. Excellent shelf life
3. Excellent stability in use
4. Fast
5. Good penetration
6. Non -film -forming
7. Low effect by organic matter
8. Ease of measurement
9. No affect on skin
10. Moderate cost
Disadvantages
1. Toxicity in recommended dilution depends on wetting agent
2. Toxic at shelf strength
3. Affected by high pH
4. Produces high foam
5. Corrosive to mild steel
* Parts Per Million (ppm)
1 lb. in 1 million lbs. = 0.01 lb. (0.15 oz.) in 1,000 gallons water (8,300 lbs.). Percentage of active material in a compound must, however, be taken into consideration. Example: If a chlorine compound contains only 30% active chlorine, amount to be used would be per 1,000 gallons water to get a 200 ppm solution: 0.15 oz. X no. of ppm needed (say 200) X 100% -- 30% = 100 oz. or 6 lbs. 4 oz. of the chlorine compound.
Mother Earth Alcohol Fuel
Chapter 1
Introduction to a Farmer's Fuel ... Alcohol
Introductory Overview of the Alcohol Production Flow Chart
A Short But Complex Story About Enzymes and Their Functions
Chapter 2
Farm Crops for Alcohol Fuel
Raw Materials
More on Raw Materials
Feedstock Handling and Storage
Chapter 3
Basic Steps in the Production of Ethyl Alcohol
More On Conversion and Fermentation
Fermentation Addendum
Alcohol Yield
Chapter 4
Control of Infection by Planned Sanitation in the Production of Fuel or Gasohol Alcohol
Chapter 5
MOTHER's Mash Recipes for Alcohol Production
Important! Read Before Making Mash
Preparing a Mash From Saccharide-rich Materials
A Handy Hydrometer Jacket
Chapter 6
Distiller's Feeds
By-product Utilization
Animal Feed By-product
More Information On By-product Utilization
Chapter 7
How the Distillation Process Works
Packed Column
Perforated Plate
Bubble Cap Plate
Solar Stills
The Reasoning Behind MOTHER's Still Design
Still Operation
Making Your First "Run"
"Economizing" Your Alcohol Production
Chapter 8
Six-Inch Column Still Plans
Three-Inch Column Still Plans
Bill of Materials
Chapter 9
Two Low-cost Backyard Stills
How To Adapt Your Automobile Engine For Ethyl Alcohol Use
Ron Novak's Do-It-Yourself Water Injection System
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
 Mother’s Alcohol Fuel Seminar
Mother’s Alcohol Fuel Seminar